Focus on Stem Cell Biology
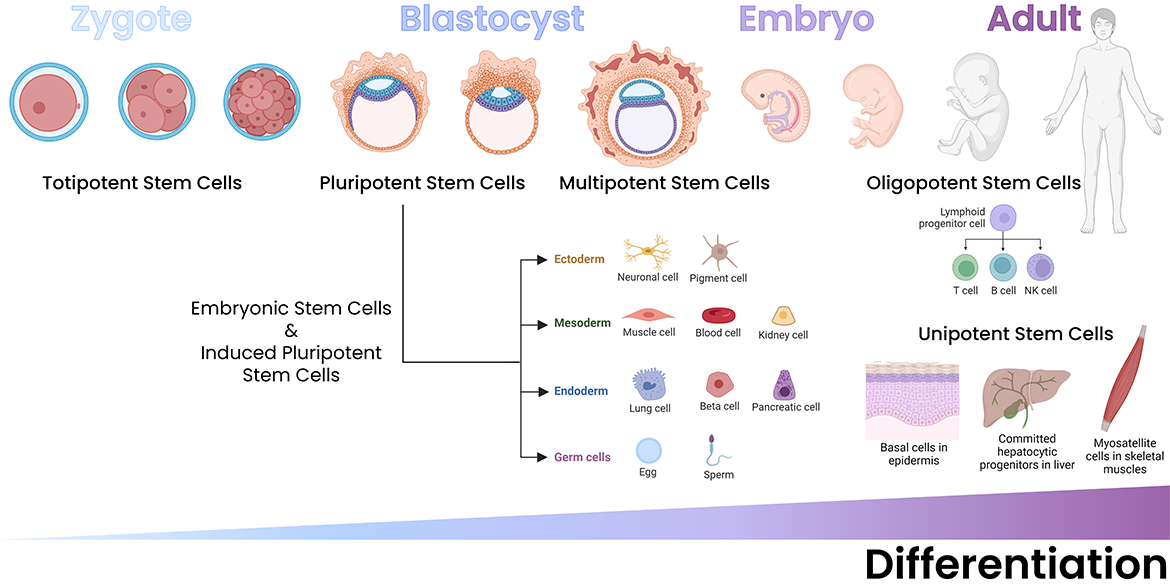
Stem cell biology is a tightly regulated process that begins during embryonic development and is present in the body in various forms through adulthood. Stem cells have the unique property of being self-renewing and either multipotent or pluripotent. Most cells in an organism are terminally differentiated, meaning that they no longer proliferate. On the other hand, pluripotent stem cells can differentiate into any tissue type (or divide to produce more stem cells, i.e., their self-renewing characteristic). Multipotent stem cells are adult stem cells that can differentiate into the terminally differentiated cells of a specific organ/tissue in which they reside.
During embryonic development, totipotent and pluripotent stem cells can both be found, depending on the stage of development. Totipotent stem cells are found immediately after the fusion of the sperm and egg. They only exist for the first few rounds of embryonic cell division and can develop into all differentiated cell/tissue types of the organism and the placenta. Pluripotent stem cells are first identified in the blastocyst stage in mammalian embryos and can develop into all differentiated cell/tissue types in an organism. The blastocyst's inner cell mass is comprised of these pluripotent stem cells.
In adulthood, somatic stem cell proliferation is a key process in recovery from tissue injury and disease. Not every adult organ contains stem cells, and this impacts the organ/tissue's ability to regenerate. Some adult organs that contain stem cells include the bone, skin, liver, intestine and skeletal muscle. Each of these niches contains stem cells that are partially differentiated, able to differentiate into organ-specific cell types. For example, hematopoietic stem cells (HSCs) are found in the bone marrow and can differentiate into all blood cell types1, whereas hepatic stem cells (i.e., oval cells or hepatocytes) have the potential to become either hepatocytes or cholangiocytes2. Furthermore, some organs, like the intestine, are frequently regenerating, while others, such as fat and muscle, can regenerate in response to tissue damage1.
Additionally, pluripotent stem cells can be laboratory-generated, and these cells are referred to as induced pluripotent stem cells, or iPSCs. Like somatic stem cells, iPSCs are typically engineered to regenerate the cell subsets of a particular organ. The factors responsible for the differentiation process are largely unique from organ to organ, although some common signaling pathways and downstream mediators do appear3. These include the Akt/AMPK axes, physical mediators of cell-cell contact such as adherens junctions and Wnt signaling, and metabolic changes.
Articles Related to Stem Cell Biology
Human Umbilical Cord Derived Mesenchymal Stem Cell Sheets for Clinical Use
With the incredible therapeutic potential of human umbilical derived mesenchymal stem cell sheets, manufacturing, preservation, and quality standards needed to be developed to ensure patient safety. Researchers developed culture and preservation methods that were able to be scaled up into a two-tiered cell bank system that maintained quality, integrity, and functionality of the mesenchymal stem cells.
The Speed Limit of the Cell Cycle
The growth and division of cells, a process termed the cell cycle, consists of four phases in eukaryotes. DNA replication occurs during the synthesis (S) phase whereas cell division occurs during the mitosis (M) phase. Two additional gap phases (G1 and G2) that occur before the S and M phases, respectively, identify any problems during DNA replication and chromosome segregation, and pause the cycle in order to allow for appropriate repairs.
The Rb-E2F Switch: Regulation of Cellular Quiescence
Cell cycle progression and proliferation have been well-studied, while much less attention has been given to cellular quiescence corresponding to the G0 phase of the cell cycle. Recent studies have determined that G0 is not a single state, but a spectrum of quiescent states associated with an active transcriptional program.
Ki-67: A Nuclear Antigen Uniquely Qualified as a Marker of Cellular Proliferation
Cellular proliferation is a fundamental biological process controlled by complex regulatory networks. Careful control of these networks is necessary for normal growth and development as well as for the body’s systemic response to infection or injury. Disruption or dysregulation of the mechanisms controlling cellular proliferation may result in inappropriate proliferation, such as in the formation and growth of tumors.
Targeting cancer stem cells with dendritic cell vaccines in melanoma
Dendritic cell vaccines targeting aldehyde dehydrogenases via peptides to ALDH1A1 and ALDH1A3 were able to reduce cancer stem cell numbers, decrease tumor growth, and increase T-cell infiltration and cytotoxic activity. This activity was enhanced with the addition of PD-L1 blockade providing additional therapeutic strategies to improve the efficacy of immune checkpoint blockade.
Sign up to receive future Focus on… features
Fortis Products for Studying Stem Cell Biology
Don’t see the see the target you are looking for? Let us know.
-
Rabbit anti-STAT5a Recombinant Monoclonal Antibody [BLR124H]
Bethyl Laboratories® Catalog # A700-124 A700-124-T A700-124CF
 ValidatedDocuments (10)
ValidatedDocuments (10)Rabbit anti-STAT5a Recombinant Monoclonal Antibody [BLR124H]
Validation Performed
Pillar 1: Independent Antibodies
Pillar 2: Complementary Assays
Target: STAT5a
Reactivity: Human, Mouse
Applications:
Platforms: COMET™
Host: Rabbit
Conjugate:
Purity:
For ordering information, see our International Distributors
Product has been discontinued
-
Rabbit anti-SMAD2 Recombinant Monoclonal Antibody [BLR130H]
Bethyl Laboratories® Catalog # A700-130 A700-130-T A700-130CF
 ValidatedDocuments (8)
ValidatedDocuments (8)Rabbit anti-SMAD2 Recombinant Monoclonal Antibody [BLR130H]
Validation Performed
Pillar 1: Independent Antibodies
Pillar 2: Complementary Assays
Pillar 4: Biological Characteristics
Target: SMAD2
Reactivity: Human, Mouse
Applications:
Platforms: COMET™
Host: Rabbit
Conjugate:
Purity:
For ordering information, see our International Distributors
Product has been discontinued
-
Rabbit anti-Rad21 Recombinant Monoclonal Antibody [BLR052F]
Bethyl Laboratories® Catalog # A700-052-T A700-052
 ValidatedDocuments (5) Citations ()
ValidatedDocuments (5) Citations ()Rabbit anti-Rad21 Recombinant Monoclonal Antibody [BLR052F]
Validation Performed
Pillar 1: Independent Antibodies
Pillar 2: Complementary Assays
Target: Rad21
Reactivity: Human, Mouse
Applications:
Host: Rabbit
Clonality: Recombinant Monoclonal
Conjugate:
Purity:
For ordering information, see our International Distributors
Product has been discontinued
-
Rabbit anti-BRD4 Recombinant Monoclonal Antibody [BL-151-6F11]
Bethyl Laboratories® Catalog # A700-005-T A700-005 A700-005CF
 ValidatedDocuments (11) Citations ()
ValidatedDocuments (11) Citations ()Rabbit anti-BRD4 Recombinant Monoclonal Antibody [BL-151-6F11]
Validation Performed
Pillar 1: Independent Antibodies
Pillar 2: Complementary Assays
Target: BRD4
Reactivity: Human, Mouse
Applications:
Platforms: PhenoImager™ HT
Host: Rabbit
Conjugate:
Purity:
For ordering information, see our International Distributors
Product has been discontinued
-
Rabbit anti-MIF Recombinant Monoclonal Antibody [BLR141J]
Bethyl Laboratories® Catalog # A700-141 A700-141-T A700-141CF
 ValidatedDocuments (7)
ValidatedDocuments (7)Rabbit anti-MIF Recombinant Monoclonal Antibody [BLR141J]
Validation Performed
Pillar 1: Independent Antibodies
Pillar 2: Complementary Assays
Target: MIF
Reactivity: Human
Applications:
Host: Rabbit
Clonality: Recombinant Monoclonal
Conjugate:
Purity:
For ordering information, see our International Distributors
Product has been discontinued
Bulk Request
References
1. Brunet, A., Goodell, M., & Rando, T. (20 July 2022) Ageing and rejuvenation of tissue stem cells and their niches https://www.nature.com/articles/s41580-022-00510-w
2. Bruno, S., Sanchez, M., Chiabotto, G., Fonsato, V., Navarro-Tableros, V., Pasquino, C., Tapparo, M., & Casmussi, G. (26 April 2021) Human Liver Stem Cells: A Liver-Derived Mesenchymal Stromal Cell-Like Population With Pro-regenerative Properties https://www.frontiersin.org/articles/10.3389/fcell.2021.644088/full
3. Callaghan, N., Durland, L., Ireland, R., Santerre, J., Simmons, C., & Huyer, L. (03 September 2022) Harnessing conserved signaling and metabolic pathways to enhance the maturation of functional engineered tissues https://www.nature.com/articles/s41536-022-00246-3


